Credits/Names: P. Illien1,2, X. Zhao2, K. K. Dey2, P. J. Butler2, A. Sen2, R. Golestanian1 1Rudolf Peierls Centre for Theoretical Physics, Oxford; 2 Penn State DMR-1420620
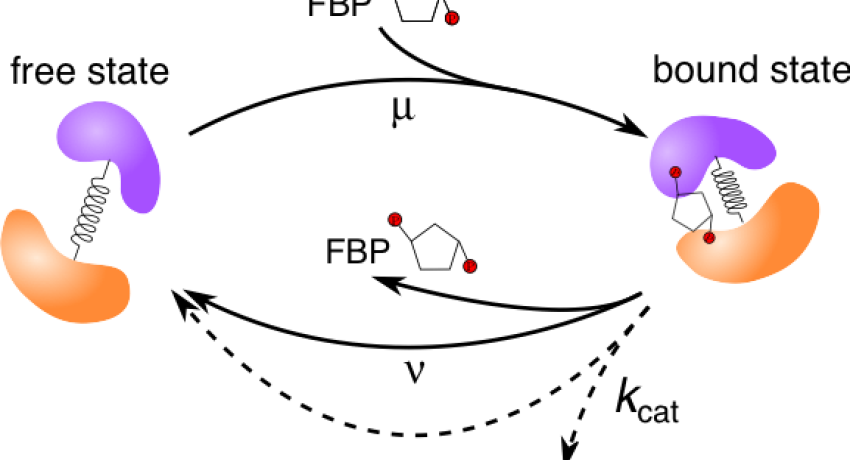
The diffusivity of enzymes is enhanced when they are catalytically active, and it was suggested that this enhancement could be correlated to their exothermicity. A MRSEC team shows that the diffusion coefficient of a model enzyme hydrodynamically coupled to its environment increases significantly when undergoing changes in conformational fluctuations in a substrate-dependent manner, and is independent of the overall turnover rate of the underlying enzymatic reaction. These findings are quantitatively supported by the team’s experiments on the endothermic and relatively slow enzyme aldolase, giving rise to a new paradigm for this phenomenon. These findings reveal that the exothermicity of the catalyzed reaction is not a necessary condition for enhanced diffusion. A better understanding of enhanced diffusion may transform our understanding of the scope of function possible for enzymes in biology.
Credits/Names: P. Illien1,2, X. Zhao2, K. K. Dey2, P. J. Butler2, A. Sen2, R. Golestanian1
1Rudolf Peierls Centre for Theoretical Physics, Oxford; 2Penn State
DMR-1420620
Download PDF Version: IRG2 Golestanian Revised.pdf
Year of Highlight: 2018
IRG: IRG 2 - Nanomotors